Work Package 1: Clinical Trial
Cryptococcal meningitis
Cryptococcal meningitis (CM) is a fungal infection that affects tissues of the brain and spinal cord. In the majority of cases, infection is caused by the fungus cryptococcus neoformans, found in animals, plants and soil. CM is a major – and neglected – opportunistic infection amongst immunosuppressed HIV positive individuals across the globe.
CM is the most common type of adult meningitis across much of Africa, and without effective treatment, infection progresses quickly. Globally, there are roughly 230,000 cases of CM and 180,000 CM-related deaths each year, the majority of which occur in sub-Saharan Africa. Tackling the high mortality rate remains challenging.
The Phase III AMBITION Trial
The current standard of care treatments for CM in sub-Saharan Africa are either (a) a 7-14-day course of amphotericin-B combined with oral antifungal tablets or (b) oral fluconazole. 10-week mortality with fluconazole treatment remains at approximately 60%, and standard treatment with amphotericin-B also has major drawbacks:
- Amphotericin-B is not widely available outside South Africa
- Amphotericin-B can cause kidney impairment and reduced blood counts
- Prolonged hospitalisation requires intensive nursing care and expensive laboratory monitoring.
- In Zimbabwe, where patients fund their own hospital admissions, such costs are prohibitive for the majority of the population.
Sustainable, cost-effective and easily-administered treatments are urgently needed.
The trial therefore investigates whether a single high dose of liposomal amphotericin-B (L-AmB, Ambisome) is as effective as 7-day amphotericin-B based therapy in averting all-cause mortality in HIV-associated CM.
The design of the Phase III trial is directly influenced by two recently completed studies, both sponsored by St. George’s University London:
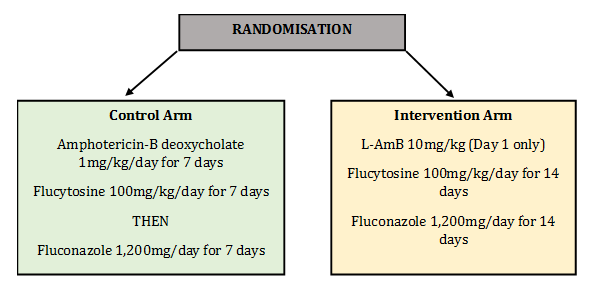
Study Design
Patients were randomised into two arms:
The latest version of the World Health Organisation Guidelines for treatment of CM can be found here:
The trial database is built and hosted by the Data Management Team at The Botswana-Harvard Partnership.
Outcomes
If successful, we anticipate that the results will be incorporated into national and international treatment guidelines within one year of trial completion.
It is also vital to ensure long-term affordability and accessibility of L-AmB in low-resource countries with the highest burden of CM. We are therefore working with the Cryptococal Meningitis Action Group (CrypoMAG), an advocacy group chaired by Co-Investigator Dr. Angela Loyse and with members from the US Centers for Disease Control, WHO, Medicines Sans Frontieres (MSF), Clinton Health Access and numerous universities and public health institutions. CryptoMAG have successfully campaigned for WHO to reinstate amphotericin-B and 5FC (flucytosine) on the essential medicines list, and are working with generic manufacturers to develop affordable preparations for Africa. Gilead, who are donating the L-AmB for the trial, are also fully supportive of the need for wider access to L-AmB for cryptococcal meningitis.
We are also involved in discussions with generic drug manufacturers who have already expressed interest in manufacturing low-cost generics for CM.


