News
March 2018
We are pleased to inform you that participants in the BLINDED arm of the trial can now ask for their treatment allocation. The delay in feeding this information back to you was due to maintaining the integrity of the statistical analysis while writing the study report. Treatment allocation lists have been sent to all Principal Investigators at GP practices in the blinded arm, so participants can request this information directly from their GP practice, or from us here at the Trial Coordinating Centre. Thank you all for your patience.
December 2017
The Vitamin D and Longevity (VIDAL) Trial feasibility study has been a huge success. We owe much of this success to you all. The hard work of our team members across the 20 sites has truly been invaluable in fulfilling our aims and objectives. Thank you all for your contribution to this research.
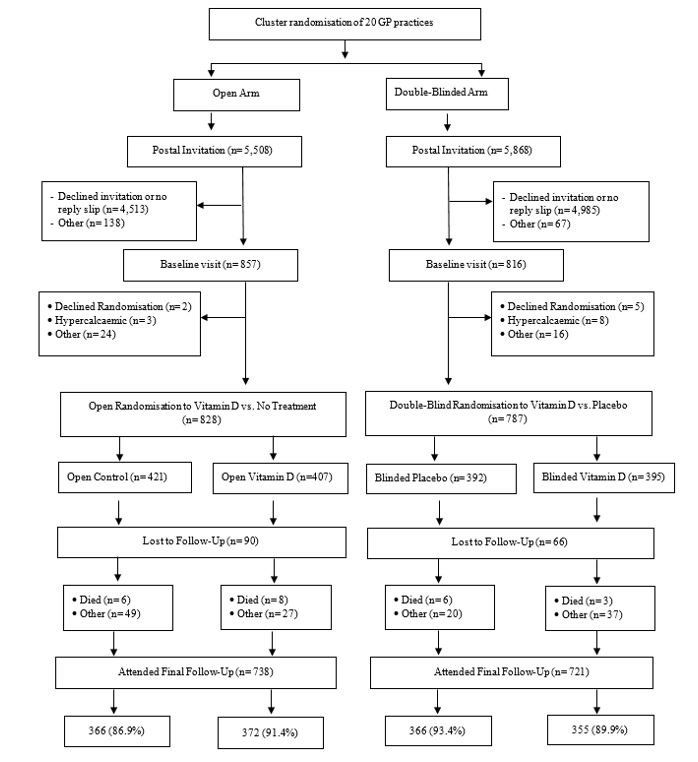
Trial Summary: High-dose vitamin D may reduce the risk of many diseases but without large randomised trials the evidence will remain inconclusive. We therefore proposed the Vitamin D and Longevity (VIDAL) trial, with 20,000 older people randomised between no vitamin D and taking vitamin D for 5 years. The VIDAL feasibility study was conducted to establish the procedures required for the main trial, including assessment of recruitment, compliance (taking study treatment as directed) and contamination (how many controls started taking vitamin D). This was done in two sets of GP practices: (i) “open” practices, where participants knew their treatment allocation (2 years of vitamin D or no treatment) and (ii) “double blind” practices, where participants and their GPs did not know whether they were taking vitamin D oil or placebo oil. We invited 11,376 men and women aged 65-84 from 20 English GP practices and 1,615 (14%) took part, see Figure 1. The majority of participants took their monthly oil for 2 years and few used vitamin supplements outside of the trial, with no marked differences between open and double blind groups. The best way to conduct the main trial will therefore depend on other considerations.
A double blind trial provides reliable evidence on effects where reporting could be influenced by you or your doctor knowing your treatment, which is particularly important for many illnesses and any side-effects of treatment. However, any long-term effects are likely to be considerably greater if treatment continues instead of stopping after 5 years when the main trial ends. An open trial is easier to conduct, and when it ends those taking vitamin D can be offered a continuing supply so that the effect of lifelong treatment can be studied for major diseases and life expectancy, which are unlikely to be affected by knowing whether you are taking vitamin D.
No funding has yet been obtained for a main trial. If and when funding becomes available, GP practices would be contacted for expressions of interest at that time.
Please feel free to contact us with any questions at: vidal@lshtm.ac.uk