Home
AMBITION (High Dose AMBISOME on a Fluconazole Backbone for Cryptococcal Meningitis Induction Therapy in sub-Saharan Africa: A Randomised Controlled Non-inferiority Trial) is a four-year project funded at €10M by the European and Developing Countries Clinical Trials Partnership, the Swedish International Development Agency and the DFID/MRC/Wellcome Trust Joint Global Health Trials Fund. It runs from 1 January 2017 – 30 June 2021 across a consortium of six African and five European partners.
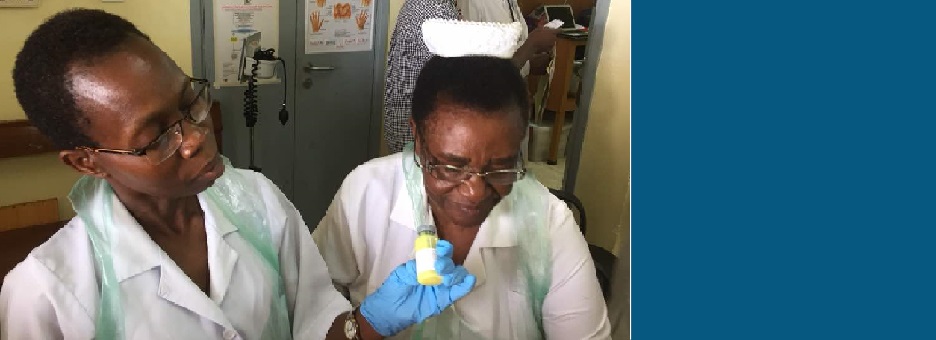
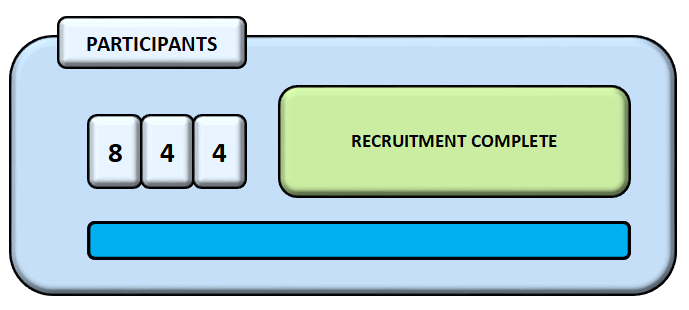
The Phase III clinical trial (AMBITION-cm) is the largest clinical trial for HIV-associated cryptococcal meningitis ever to be conducted. The trial completed recruitment in February 2021. We are grateful to everyone who has enabled us to achieve this; our exceptionally dedicated study teams who have provided an outstanding level of care, all participants and their families, and other senior investigators and individuals who have contributed in numerous ways including participating in the TSC and DMC, lending expertise to our portfolio of capacity-strengthening events, developing our Electronic Data Capture Tool and undertaking data analysis.
A number of other activities run alongside the trial, including economic cost-effectiveness analyses, laboratory-based sub-studies and capacity building events. AMNET (African Meningitis Network) has been established to provide a solid framework to facilitate future clinical trials to investigate urgently-needed novel treatments and diagnostics strategies aimed at reducing the morbidity and mortality due to meningitis in Africa.
AMBITION is co-ordinated by The London School of Hygiene and Tropical Medicine, UK. African collaborating institutions (where participants were recruited, in conjunction with local hospitals) are shown below.
The AMBITION consortium is extremely grateful to the following funders:
- The European and Developing Countries Clinical Trials Partnership (EDCTP)
- The Swedish International Development Agency
- The DFID/MRC/Wellcome Trust Joint Global Health Trials Scheme
…and to Gilead, who has donated the AmBisome for the clinical trial.