LEOPARD
LEOPARD:

Little is known about how individuals and their caregivers experience the clinical trial process when suffering from a life-threatening illness. The AMBITION trial therefore provides a unique setting to conduct a qualitative study of the research experience. This is because participants will be unwell and may be confused on enrolment, mortality will be roughly 25-40% and the trial is conducted through a consortium composed of six African and five European institutions.
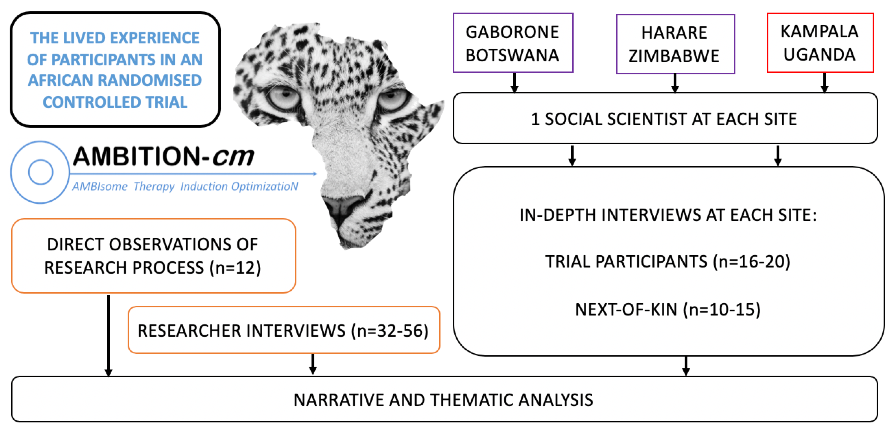
The LEOPARD study is an in-depth qualitative study, nested within AMBITION, with the aim to document the AMBITION participant experience. Using ethnography and narrative enquiry the team will develop an understanding of how trial delivery could be improved. The perspective of the participant, their next-of-kin, and a broad range of researchers will be sought. The study is led by Dr. David Lawrence, supervised by Profs. Joe Jarvis and Janet Seeley.
A team of social scientists from each AMBITION site, led by Dr. Lawrence, have developed a uniform methodology. At three of the AMBITION sites: Gaborone, Harare and Kampala, in-depth interviews are taking place with AMBITION participants and their next-of-kin. Data collection follows a narrative approach, focusing on the concept of time. In addition, the perspectives of researchers from Africa and Europe are explored using in-depth interviews and direct observation which will be subject to thematic analysis. The ultimate objective of the study is to use the data to improve both this trial and similar trials in the future.

Gaborone:
- PI – Dr. David Lawrence
- Katlego Tsholo

Kampala:
- PI – Dr. David Meya
- Agnes Ssali, Social Scientist, MRC/UVRI/LSHTM Entebbe
- Georgina Nabaggala, Social Scientist, MRC/UVRI/LSHTM Entebbe

Harare:
- PI – Prof. Rati Ndhlovu
- Zivai Mupambireyi, Social Scientist, Centre for Sexual Health and HIV Research Zimbabwe


