PAGE separation of small RNAs
For the resolution of RNAs between 15 and 500 nucleotides in length, depending on the percentage acrylamide used.
Materials
Formamide buffer (0.01M EDTA, 0.1% bromophenol blue, deionised formamide)
Gel set-up
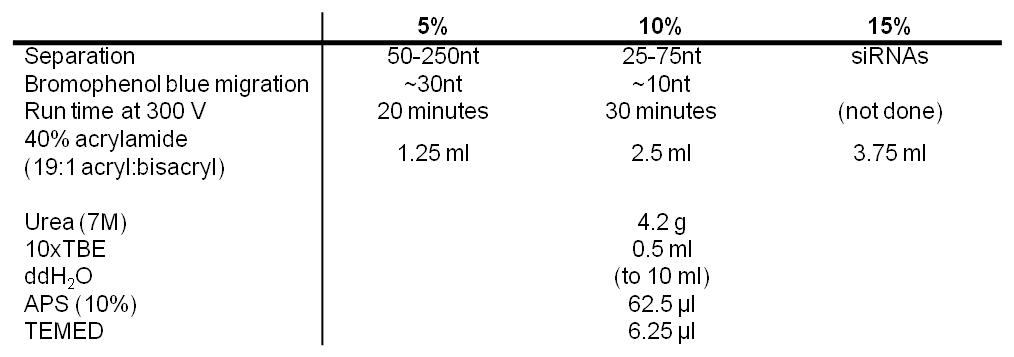 NB: bromophenol blue migration and run time based on an 8×5 cm mini-gel
NB: bromophenol blue migration and run time based on an 8×5 cm mini-gel
- Put together the running assembly, fill with 0.5x TBE, remove the comb and clean the wells with running buffer
- Pre-run the gel for 1 hour at 300 V
Sample preparation & gel running
- Resuspend 1 μg RNA in 2.5 μl ddH2O (RNAse-free) and add 7.5 μl formamide buffer
- Denature at 65°C for 15 minutes, spin briefly, place on ice; load samples taking care not to overflow into neighbouring wells
- Run gel at 300 V or until the dye front runs off the gel
Staining & transfer
- Remove the gel from the assembly and place in 0.5x TBE containing 50ng/ml ethidium bromide for 10 minutes; de-stain in 0.5x TBE for 10 minutes
- Transfer to positively charged nylon membrane (e.g. Zeta membrane, Bio-Rad) using a semi-dry electroblotter (e.g. transblotter, Bio-Rad)
- Pre-soak the membrane and two pieces of extra thick filter paper in 0.5x TBE for 10 minutes
- Set-up the transfer stack (bottom: filter paper, membrane, gel, filter paper: top) and transfer for 1 hour at 250 mA
- Cross-link in a Stratalinker or similar
Pre-hybridise and hybridise as per standard northern protocol
